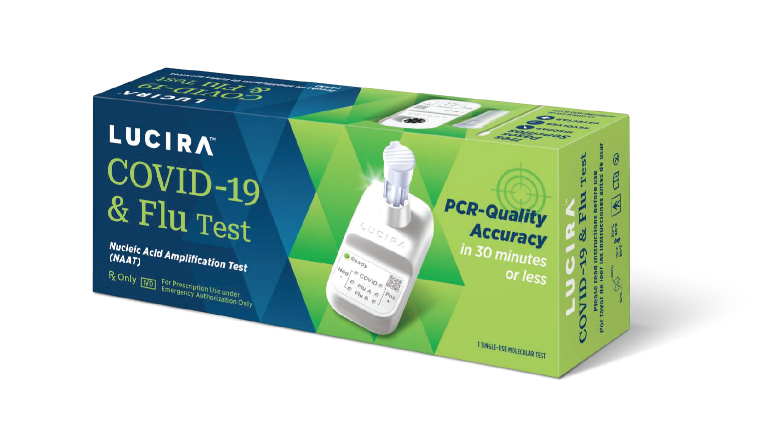
NOW AVAILABLE!
The Only FDA Authorized Equipment-Free Molecular COVID & Flu Test
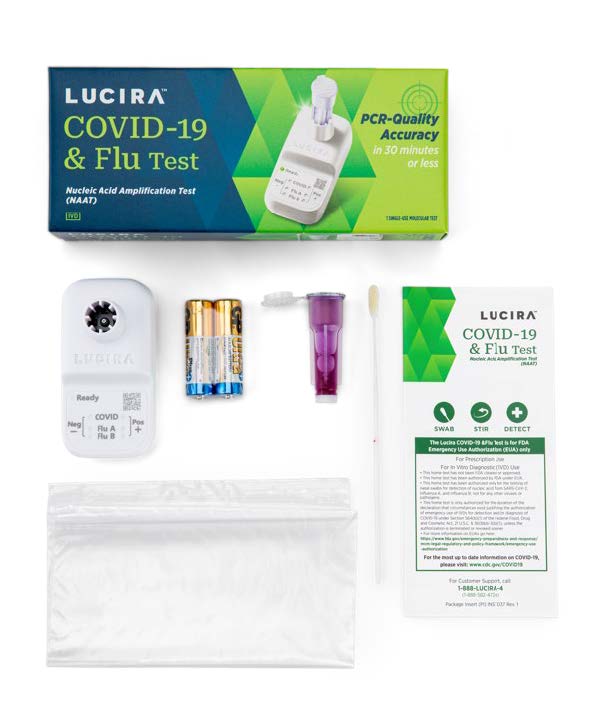
- First and only FDA authorized equipment-free molecular COVID & Flu Test
- PCR-quality accuracy
- 3 viruses, 1 swab, 30 minutes or less
- No capital equipment, calibration or external controls required
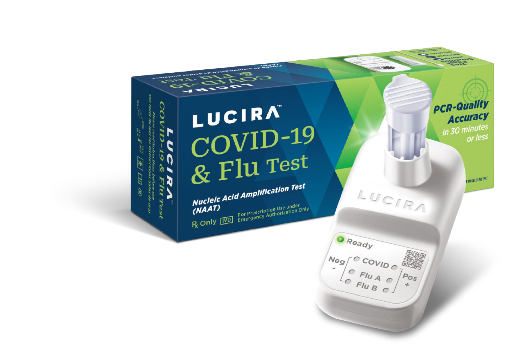
 Item # 910170(single unit):..................................................$71.31
Item # 910170(single unit):..................................................$71.31Item # 910171(24 pack):.......................................................$1,626($67.75/ unit)
Bring Testing Closer to Patients: LUCIRA Combines PCR-Quality Accuracy with Flexibility & Simplicity of Rapid Tests
Test anytime, anywhere*
No instrument, reader, power source or internet connection
Easy-to-use*
No capital equipment, calibration, or external controls required
Swab
Shallow anterior nasal swab
Stir
No droppers, mixing, or shaking
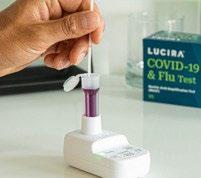

Detect
Positive or negative light; simple interpretation
COVID-19 and Flu A/B Reimbursement at Point-of-Care (POC) or Lab
| TEST | CPT Code | 2021 Medicare Reimbursement Rate |
|---|---|---|
| COVID-19 Molecular* | 87635 | $51.31 |
| COVID-19 & Flu A/B Molecular* | 87636 | $142.63 |
| COVID-19 Antigen | 87426 | $35.33 |
| COVID-19 & Flu A/B Antigen | 87428 | $63.59 |
Source: CMS https://www.cms.gov/files/document/mac-covid-19-test-pricing.pdf
* Molecular codes used for tests that amplify viral genetic material: includes lab-based PCR assays, point-of-care PCR or other molecular assays, and at-home molecular assays used at POC including Lucira tests
RESOURCE & LINKS
CPT Code: 97636
UPC:

LOINC Codes:
• Coding System Version 2.74-pre
•
https://loinc.org/100972-9/
for the OBR
•
https://loinc.org/96986-5/
for the COVID OBX
•
https://loinc.org/100973-7/
for the Flu A OBX
•
https://loinc.org/100974-5/
for the Flu B OBX